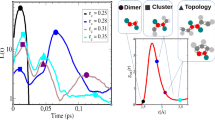
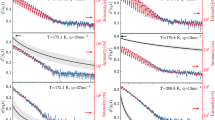
Abstract
HYDROGEN bonds play a crucial role in the behaviour of water1–4; their spatial patterns and fluctuations characterize the structure and dynamics of the liquid5–7. The processes of breaking and making hydrogen bonds in the condensed phase can be probed indirectly by a variety of experimental techniques8, and more quantitative information can be obtained from computer simulations9. In particular, simulations have revealed that on long timescales the relaxation behaviour of hydrogen bonds in liquid water exhibit non-exponential kinetics7,10–13, suggesting that bond making and breaking are not simple processes characterized by well defined rate constants. Here we show that these kinetics can be understood in terms of an interplay between diffusion and hydrogen-bond dynamics. In our model, which can be extended to other hydrogen-bonded liquids, diffusion governs whether a specific pair of water molecules are near neighbours, and hydrogen bonds between such pairs form and persist at random with average lifetimes determined by rate constants for bond making and breaking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Teixeira, J. J. Physique IV C1, 3, 162–169 (1993).
Eisenberg, D. & Kauzmann, W. The Structure and Properties of Water (Oxford Univ. Press, New York, 1969).
Franks, F. (ed.) Water Science Reviews Vols 1–5 (Cambridge Univ. Press, 1985–90).
Stanley, H. E. & Ostrowzky, N. (eds) Correlations and Connectivity, Geometric Aspects of Physics, Chemistry and Biology (Kluwer Academic, Dordrecht, 1990).
Stillinger, F. H. Adv. chem. Phys. 31, 1–101 (1975).
Stillinger, F. H. Science 209, 451–457 (1980).
Ohmine, I. & Tanaka, H. Chem. Rev. 93, 2545–2566 (1993).
Dore, J. C. & Teixeira, J. (eds) Hydrogen-Bonded Liquids (Kluwer Academic, Dordrecht, 1991).
Ladanyi, B. M. & Skaf, M. S. A. Rev. Chem. 44, 335–368 (1993).
Belch, A. C. & Rice, S. A. J. chem. Phys. 86, 5676–5682 (1987).
Sciortino, F., Poole, P. H., Stanley, H. E. & Havlin, S. Phys. Rev. Lett. 64, 1686–1689 (1990).
Zichi, D. A. & Rossky, P. J. J. chem. Phys. 84, 2814–2822 (1986).
Luzar, A. & Chandler, D. in Hydrogen Bond Networks (eds Bellisent-Funel, M. C. & Dore, J. C.) 239–246 (Kluwer Academic, Dordrecht, 1994).
Chandler, D. Introduction to Modern Statistical Mechanics (Oxford Univ. Press, New York, 1987).
Chandler, D. J. chem. Phys. 68, 2959–2970 (1978).
Berne, B. J. in Multiple Time Scales (eds Brackbill, J. U. & Cohen, B. I.) 419–436 (Academic, New York, 1985).
Saito, S. & Ohmine, I. J. chem. Phys. 102, 3566–3579 (1995).
Ferrario, M., Haughley, M., McDonald, I. R. & Klein, M. L. J. chem. Phys. 93, 5156–5166 (1990).
Luzar, A. & Chandler, D. J. chem. Phys. 98, 8160–8173 (1993).
Soper, A. K. & Phillips, M. G. Chem. Phys. 107, 47–60 (1986).
Teixeira, J., Bellissent-Funel, M.-C., Chen, S. H. & Dianoux, A. J. Phys. Rev. A31, 1913–1917 (1985).
Bratos, S. & Leicknam, J.-C. J. chem. Phys. 103, 4887–4893 (1995).
Berendsen, H. J. C., Postma, J. P. M., van Gusteren, W. F. & Hermans, J. in Intermolecular Forces (ed. Pullman, B.) 331–342 (Reidel, Dordrecht, 1981).
Allan, M. P. & Tildesley, D. J. Computer Simulation of Liquids (Clarendon, Oxford, 1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luzar, A., Chandler, D. Hydrogen-bond kinetics in liquid water. Nature 379, 55–57 (1996). https://doi.org/10.1038/379055a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/379055a0
This article is cited by
-
Electrofreezing of liquid water at ambient conditions
Nature Communications (2024)
-
Impact of hierarchical water dipole orderings on the dynamics of aqueous salt solutions
Nature Communications (2023)
-
Femtosecond proton transfer in urea solutions probed by X-ray spectroscopy
Nature (2023)
-
The collective burst mechanism of angular jumps in liquid water
Nature Communications (2023)
-
Theoretical Insight into the Effect of Steam Temperature on Heavy Oil/Steam Interface Behaviors Using Molecular Dynamics Simulation
Journal of Thermal Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.