ABSTRACT
Purpose
The objectives of the study was to develop a dissolution test method that can be used to predict the oral absorption of montelukast sodium, and to establish an in vitro/in vivo correlation (IVIVC) using computer simulations.
Methods
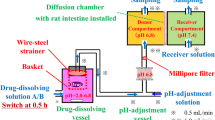
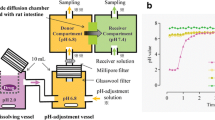
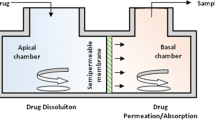
Drug solubility was measured in different media. The dissolution behaviour of montelukast sodium 10 mg film coated tablets was studied using the flow-through cell dissolution method following a dynamic pH change protocol, as well as in the USP Apparatus 2. Computer simulations were performed using GastroPlus™. Biorelevant dissolution media (BDM) prepared using bile salts and lecithin in buffers was used as the dissolution media, as well as the USP simulated intestinal fluid (SIF) pH 6.8 and blank FaSSIF pH 6.5. Dissolution tests in the USP Apparatus 2 were performed under a constant pH condition, while the pH range used in the flow through cells was pH 2.0 to 7.5. The in vitro data were used as input functions into GastroPlus™ to simulate the in vivo profiles of the drug.
Results
The solubility of montelukast sodium was low at low pH, but increased as the pH was increased. There was no significant difference in solubility in the pH range of 5.0 to 7.5 in blank buffers, but the drug solubility was higher in biorelevant media compared with the corresponding blank buffers at the same pH. Using the flow through cells, the dissolution rate was fast in simulated gastric fluid containing 0.1% SLS. The dissolution rate slowed down when the medium was changed to FaSSIF pH 6.5 and increased when the medium was changed to FaSSIF medium at pH 7.5. In the USP Apparatus 2, better dissolution was observed in FaSSIF compared with the USP buffers and blank FaSSIF with similar pH values. Dissolution was incomplete with less than 10% of the drug dissolved in the USP-SIF, and was practically non existent in blank FaSSIF pH 6.5. The in vitro results of the dynamic dissolution test were able to predict the clinical data from a bioavailability study best.
Conclusions
Dynamic dissolution testing using the flow through cell seems to be a powerful tool to establish in vitro/in vivo correlations for poorly soluble drugs as input function into GastroPlus.





Similar content being viewed by others
References
T. R. Jones, M. Labelle, M. Belley, E. Champion, L. Charette, J. Evans, A. W. Ford-Hutchinson, J. Y. Gauthier, A. Lord, P. Masson et al. Pharmacology of montelukast sodium (Singulair), a potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 73:191–201 (1995).
R. M. H. Thibert, S. D. Clas, D. R. Meisner, and E. B. Vadas. Characterization of the self-association properties of a leukotrieneD 4 receptor antagonist, MK-0476. Int. J. Pharm. 134:59–70 (1996), DOI 10.1016/0378-5173(96)04435-3.
J. B. Dressman, G. L. Amidon, C. Reppas, and V. P. Shah. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 15:11–22 (1998) DOI 10.1023/A:1011984216775.
FDA-CDER. Guidance for Industry, Dissolution Testing of Immediate release Solid Oral Dosage Forms, U.S. Department of Health and Human Services, Food and Drug Administration, 1997.
J. Emami. In vitro–in vivo correlation: from theory to applications. J. Pharm. Pharm. Sci. 9:169–189 (2006).
V. P. Shah, and L. J. Lesko. Current challenges and future regulatory directions in in vitro dissolution. Drug Inform. J. 29:885–891 (1995).
J. B. Dressman, G. L. Amidon, and D. Fleisher. Absorption potential: estimating the fraction absorbed for orally administered compounds. J. Pharm. Sci. 74:588–589 (1985) DOI 10.1002/jps.2600740523.
W. N. Charman, C. J. Porter, S. Mithani, and J. B. Dressman. Physiochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J. Pharm. Sci. 86:269–282 (1997) DOI 10.1021/js960085v.
D. Horter, and J. B. Dressman. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 46:75–87 (2001) DOI 10.1016/S0169-409X(00)00130-7.
L. X. Yu, E. Lipka, J. R. Crison, and G. L. Amidon. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv. Drug Deliv. Rev. 19:359–376 (1996) DOI 10.1016/0169-409X(96)00009-9.
S. Willmann, W. Schmitt, J. Keldenich, J. Lippert, and J. B. Dressman. A physiological model for the estimation of the fraction dose absorbed in humans. J. Med. Chem. 47:4022–4031 (2004) DOI 10.1021/jm030999b.
H. Cai, C. Stoner, A. Reddy, S. Freiwald, D. Smith, R. Winters, C. Stankovic, and N. Surendran. Evaluation of an integrated in vitro-in silico PBPK (physiologically based pharmacokinetic) model to provide estimates of human bioavailability. Int. J. Pharm. 308:133–139 (2006) DOI 10.1016/j.ijpharm.2005.11.002.
H. Wei, and R. Lobenberg. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur. J. Pharm. Sci. 29:45–52 (2006) DOI 10.1016/j.ejps.2006.05.004.
B. Agoram, W. S. Woltosz, and M. B. Bolger. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 50(Suppl 1):S41–67 (2001) DOI 10.1016/S0169-409X(01)00179-X.
SimulationsPlus. GastroPlus Manual. Lancaster, USA, 2006.
USP. United States Pharmacopeia 29/National Formulary 24. The USP, Rockville, MD, 2006.
S. A. Qureshi, G. Caillé, R. Brien, R. Piccirilli, V. Yu, and I. J. McGilveray. Application of flow-through dissolution method for the evaluation of oral formulations of nifedipine. Drug Dev. Ind. Pharm. 20:1869–1882 (1994), DOI 10.3109/03639049409050214.
K. Thoma, and I. Ziegler. Development of an automated flow-through dissolution system for poorly soluble drugs with poor chemical stability in dissolution media. Pharmazie. 53:784–790 (1998).
M. Marques. Dissolution Media Simulating fasted and Fed States, Dissolution Technologies Vol. 16, 2004.
E. Galia, E. Nicolaides, D. Horter, R. Lobenberg, C. Reppas, and J. B. Dressman. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm. Res. 15:698–705 (1998) DOI 10.1023/A:1011910801212.
L. V. Allen, N. G. Popovich, and H. C. Ansel. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery systems. Lippincott Williams and Wilkins, Philadelphia, 2005.
R. Lobenberg, and G. L. Amidon. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 50:3–12 (2000) DOI 10.1016/S0939-6411(00)00091-6.
S. S. Davis, J. G. Hardy, and J. W. Fara. Transit of pharmaceutical dosage forms through the small intestine. Gut. 27:886–892 (1986) DOI 10.1136/gut.27.8.886.
J. Fallingborg, P. Pedersen, and B. A. Jacobsen. Small intestinal transit time and intraluminal pH in ileocecal resected patients with Crohn’s disease. Dig. Dis. Sci. 43:702–705 (1998) DOI 10.1023/A:1018893409596.
P. Mojaverian, K. Chan, A. Desai, and V. John. Gastrointestinal transit of a solid indigestible capsule as measured by radiotelemetry and dual gamma scintigraphy. Pharm. Res. 6:719–724 (1989) DOI 10.1023/A:1015998708560.
J. Fallingborg. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 46:183–196 (1999).
H. Cheng, J. A. Leff, R. Amin, B. J. Gertz, M. De Smet, N. Noonan, J. D. Rogers, W. Malbecq, D. Meisner, and G. Somers. Pharmacokinetics, bioavailability, and safety of montelukast sodium (MK-0476) in healthy males and females. Pharm. Res. 13:445–448 (1996) DOI 10.1023/A:1016056912698.
L. Shargel, and A. B. C. Yu. Applied Biopharmaceutics and Pharmacokinetics. McGraw-Hill, New York, 1999.
J. W. Moore, H. H, Flanner. Mathematical Comparison of Dissolution Profiles, Pharmaceutical Technology, Vol. 6, 1996, pp. 64–74.
FDA-CDER. Guidance for Industry Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. In U.S. Department of Health and Human Services Food and Drug Administration (ed.), 1997.
J. Jinno, D. Oh, J. R. Crison, and G. L. Amidon. Dissolution of ionizable water-insoluble drugs: the combined effect of pH and surfactant. J. Pharm. Sci. 89:268–274 (2000) DOI 10.1002/(SICI)1520-6017(200002)89:2<268::AID-JPS14>3.0.CO;2-F.
S. D. Mithani, V. Bakatselou, C. N. TenHoor, and J. B. Dressman. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm. Res. 13:163–167 (1996) DOI 10.1023/A:1016062224568.
J. R. Crison, N. D. Weiner, and G. L. Amidon. Dissolution media for in vitro testing of water-insoluble drugs: effect of surfactant purity and electrolyte on in vitro dissolution of carbamazepine in aqueous solutions of sodium lauryl sulfate. J. Pharm. Sci. 86:384–388 (1997) DOI 10.1021/js960105t.
M. A. Hammad, and B. W. Muller. Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 46:361–367 (1998) DOI 10.1016/S0939-6411(98)00037-X.
Y. Wu, D. O. Kildsig, and E. S. Ghaly. Effect of hydrodynamic environment on tablets dissolution rate. Pharm. Dev. Technol. 9:25–37 (2004) DOI 10.1081/PDT-120027415.
C. Y. Perng, A. S. Kearney, N. R. Palepu, B. R. Smith, and L. M. Azzarano. Assessment of oral bioavailability enhancing approaches for SB-247083 using flow-through cell dissolution testing as one of the screens. Int. J. Pharm. 250:147–156 (2003) DOI 10.1016/S0378-5173(02)00521-5.
V. H. Sunesen, B. L. Pedersen, H. G. Kristensen, and A. Mullertz. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur. J. Pharm. Sci. 24:305–313 (2005) DOI 10.1016/j.ejps.2004.11.007.
J. J. Zhao, J. D. Rogers, S. D. Holland, P. Larson, R. D. Amin, R. Haesen, A. Freeman, M. Seiberling, M. Merz, and H. Cheng. Pharmacokinetics and bioavailability of montelukast sodium (MK-0476) in healthy young and elderly volunteers. Biopharm. Drug Dispos. 18:769–777 (1997) DOI 10.1002/(SICI)1099-081X(199712)18:9<769::AID-BDD60>3.0.CO;2-K.
S. K. Balani, X. Xu, V. Pratha, M. A. Koss, R. D. Amin, C. Dufresne, R. R. Miller, B. H. Arison, G. A. Doss, M. Chiba, A. Freeman, S. D. Holland, J. I. Schwartz, K. C. Lasseter, B. J. Gertz, J. I. Isenberg, J. D. Rogers, J. H. Lin, and T. A. Baillie. Metabolic profiles of montelukast sodium (Singulair), a potent cysteinyl leukotriene1 receptor antagonist, in human plasma and bile. Drug Metab. Dispos. 25:1282–1287 (1997).
M. Chiba, X. Xu, J. A. Nishime, S. K. Balani, and J. H. Lin. Hepatic microsomal metabolism of montelukast, a potent leukotriene D4 receptor antagonist, in humans. Drug Metab. Dispos. 25:1022–1031 (1997).
Acknowledgments
This work was supported by a collaborative research grant from NSERC and Merck Frosst Canada Inc. We would like to thank Simulations Plus for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okumu, A., DiMaso, M. & Löbenberg, R. Dynamic Dissolution Testing To Establish In Vitro/In Vivo Correlations for Montelukast Sodium, a Poorly Soluble Drug. Pharm Res 25, 2778–2785 (2008). https://doi.org/10.1007/s11095-008-9642-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9642-z