Abstract
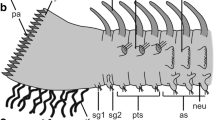
The mechanisms by which light elicits a phototactic response in sponge larvae remain poorly understood. Here we investigate histological and behavioral aspects of the photoresponse in parenchymella larvae of three demosponges. Two species are photonegative during their entire larval life, while the other, initially photopositive, becomes photonegative only after swimming in the laboratory for 4 h to 6 h. All larvae are bullet-shaped, with a uniformly ciliated surface, except at their posterior end, which is unciliated but surrounded by a distinctive ring of long cilia, the tuft. The short cilia beat metachronally, generating the thrust to move the larva forward with clockwise rotation. The long cilia of the tuft do not beat metachronally and are apparently more involved in maneuvering than in the generation of thrust. Transmission electron microscopy revealed in one species that the axoneme of the short cilia contains a distinctive "9×3+2" microtubule pattern at its base, but the presence of such an arrangement in cilia of the tuft remains uncorroborated. Nevertheless, the differences in beating characteristics between the monociliated cells of the tuft and those in the rest of the body correspond to other cytological differences. Cilia of the tuft have a type-I basal body, a large basal foot, and a branched rootlet, whereas the remaining cilia have a type-II basal body, a smaller and simpler basal foot, and an unbranched rootlet. Furthermore, the cells forming the tuft have a characteristic distal protrusion filled with pigments and mitochondria. Several of these traits suggest that the monociliated cells of the tuft are involved in the larval photoresponse both as sensors and effectors. Drastic changes in light intensity have no effect on the beating of the short cilia. In contrast, they cause a predictable and instantaneous movement of each cilium in the tuft, triggering expansions and contractions of either a part or the entire tuft, which in turn alters the direction of swimming. Observations on free-swimming larvae suggest that the tuft works as a passive light-sensitive rudder in both photonegative species that contract their posterior cilia under high irradiance and in photopositive species that expand their cilia under high irradiance. However, in photonegative larvae that expand the tuft under high irradiance, an active ciliary coordination by the larva needs to be invoked to explain a deviation of the swimming trajectory.








Similar content being viewed by others
Notes
The term "cilia" is hereafter used to refer to eukaryotic organelles that are structurally characterized by an essentially identical arrangement of microtubules. Following Nielsen (1987), this definition covers the undulating cilium of many protists and sperm cells to the planar cilium of vertebrate multiciliated cells. The term "flagellum" is used for simpler structures found in the bacteria, which lack microtubules.
References
Amano S, Hori I (1994) Metamorphosis of a demosponge. I. Cell and structure of swimming larva. Invertebr Reprod Dev 25:193–204
Amano S, Hori I (1996) Transdifferentiation of larval flagellated cells to choanocytes in the metamorphosis of the demosponge Haliclona permollis. Biol Bull 190:161–172
Bergquist PR, Sinclair ME (1968) The morphology and behavior of larvae of some intertidal sponges. N Z J Mar Freshw Res 2:426–437
Bergquist PR, Sinclair ME, Green CR, Silyn-Roberts H (1979) Comparative morphology and behaviour of larvae of Demospongiae. In: Lévi C, Boury-Esnault N (eds) Biologie des spongiaires. CNRS, Paris
Blumer MJ (1998) Alterations of the eyes of Carinaria lamarcki (Gastropoda, Heteropoda) during the long pelagic cycle. Zoomorph 118:183–194
Blumer MJF, Salvini-Plawen L, Kikinger R, Büchinger T (1995) Ocelli in a Cnidaria polyp: the ultrastructure of the pigment spot in Stylocoronella riedli (Scyphozoa, Stauromedusae). Zoomorph 115:221–227
Bourget E, DeGuise J, Daigle G (1994) Scales of substratum heterogeneity, structural complexity, and early establishment of a marine epibenthic community. J Exp Mar Biol Ecol 181:31–51
Boury-Esnault N (1976) Ultrastructure de la larve parenchymella d'Hamigera hamigera (Schmidt) (Démosponge, Poecilosclerida). Origine des cellules grises. Cah Biol Mar 17:9–20
Brill B (1973) Untersuchungen zur ultrastruktur der choanocyte von Ephydatia fluviatilis L. Z Zellforsch Mikrosk Anat 144:231–245
Chia F-S, Koss R (1979) Fine structural studies of the nervous system and the apical organ in the planula larva of the sea anemone Anthopleura elegantissima. J Morphol 160:275–298
Chia F-S, Buckland-Nicks J, Young CM (1983) Locomotion of marine invertebrate larvae: a review. Can J Zool 62:1205–1222
Cronin TW (1986) Photoreception in marine invertebrates. Am Zool 26:403–415
Eakin RM (1963) Lines of evolution of photoreceptors. In: Mazia D, Tyler A (eds) General physiology of cell specialization. McGraw-Hill, New York
Eakin RM, Hermans CO (1988) Eyes. Microf Mar 4:135–156
Evans CW (1977) The ultrastructure of larvae from the marine sponge Halichondria moorei Bergquist (Porifera, Demospongiae). Cah Biol Mar 18:427–433
Fell PE (1974) Porifera. In: Giese AC, Pearse JS (eds) Acoelomate and pseudocoelomate metazoans. Academic Press, New York and London
Forward RB, Cronin TW, Stearns DE (1984) Control of diel vertical migration: photoresponses of a larval crustacean. Limnol Oceanogr 29:146–154
Foster KW, Saranak J, Patel N, Zarilli G, Okabe M, Kline T, Nakanishi K (1984) A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eucaryote Chlamydomonas. Nature 311:756–759
Fry WG (1971) The biology of larvae of Ophlitaspongia seriata from two North Wales populations. Fourth European Mar Biol Symp:155–178
Gordon RE (1982) Three-dimensional organization of microtubules and microfilaments of the basal apparatus of ciliated respiratory epithelium. Cell Motil 4:385–391
Hard R, Rieder CL (1983) Muciliary transport in newt lungs: the ultrastructure of the ciliary apparatus in isolated epithelial sheets and in functional triton-extracted models. Tissue Cell 15:227–243
Harz H, Hegemann P (1991) Rhodopsin-regulated calcium currents in Chlamydomonas. Nature 351:489–491
Hegemann P (1997) Vision in microalgae. Planta 203:265–274
Hills JM, Thomason JC, Milligan JL, Richardson M (1998) Do barnacle larvae respond to multiple settlement cues over a range of spatial scales? Hydrobiologia 375/376:101–111
Jaeckle WB (1995) Transport and metabolism of alanine and palmitic acid by field-collected larvae of Tedania ignis (Porifera, Demospongiae): estimated consequences of limited label translocation. Biol Bull 189:159–167
Kamiya R, Witman GB (1984) Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol 98:97–107
Kaye HR, Reiswig HM (1991) Sexual reproduction in four Caribbean commercial sponges. III. Larval behaviour, settlement and metamorphosis. Invertebr Reprod Dev 19:25–35
Konstantinova MI (1966) Characteristic of movement of pelagic larvae of marine invertebrates. Dokl Akad Nauk SSSR 170:726–729
Lévi C (1964) Ultrastructure de la larve parenchymella de Démosponge. I. Mycale contarenii (Martens). Cah Biol Mar 5:97–104
Leys SP, Degnan BM (2001) Cytological basis of photoresponsive behavior in a sponge larva. Biol Bull 201:323–338
Leys SP, Cronin TW, Degnan BM, Marshall JN (2002) Spectral sensitivity in a sponge larva. J Comp Physiol [A] 188:199–202
Maldonado M, Bergquist PR (2002) Phylum Porifera. In: Young CM, Sewell MA, Rice ME (eds) Atlas of marine invertebrate larvae. Academic Press, San Diego
Maldonado M, Uriz MJ (1998) Microrefuge exploitation by subtidal encrusting sponges: patterns of settlement and post-settlement survival. Mar Ecol Prog Ser 174:141–150
Maldonado M, Young CM (1996) Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Mar Ecol Prog Ser 138:169–180
Maldonado M, Young CM (1999) Effects of the duration of the larval life on post-larval stages of the demosponge Sigmadocia caerulea. J Exp Mar Biol Ecol 232:9–21
Maldonado M, George SB, Young CM, Vaquerizo I (1997) Depth regulation in parenchymella larvae of a demosponge: relative roles of skeletogenesis, biochemical changes and behavior. Mar Ecol Prog Ser 148:115–124
Nielsen C (1987) Structure and function of metazoan ciliary bands and their phylogenetic significance. Acta Zool (Stockholm) 68:205–262
Pitelka DR (1974) Basal bodies and root structures. In: Sleigh MA (ed) Cilia and flagella. Academic Press, New York
Raimondi PT (1990) Patterns, mechanisms, consequences of variability in settlement and recruitment of an intertidal barnacle. Ecol Monogr 60:283–309
Sanderson MJ (1984) Cilia. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument. 1. Invertebrates. Springer, Berlin Heidelberg New York
Sandoz D, Chailley B, Boisvieux-Ulrich E, Lemullois M, Laine M-C, Bautista-Harris G (1988) Organization and functions of cytoskeleton in metazoan ciliated cells. Biol Cell 63:183–194
Sarà M, Vacelet J (1973) Ecologie des Démosponges. In: Grassé PP (ed) Spongiaires. Anatomie, physiologie, systématique, ecologie. Masson, Paris
Saranak J, Foster KW (1997) Rhodopsin guides fungal phototaxis. Nature 387:465–466
Singla CL (1974) Ocelli of Hydromedusae. Cell Tissue Res 149:413–429
Thomas MB, Freeman G, Martin VJ (1987) The embryonic origin of neurosensory cells and the role of nerve cells in metamorphosis in Phialidium gregarium (Cnidaria, Hydrozoa). Invert Biol 11:265–287
Uriz MJ, Maldonado M, Turon X, Martí R (1998) How do reproductive output, larval behaviour, and recruitment contribute to adult spatial patterns in Mediterranean encrusting sponges? Mar Ecol Prog Ser 167:137–148
Uriz MJ, Turon X, Becerro MA (2001) Morphology and ultrastructure of the swimming larvae of Crambe crambe (Demospongiae, Poecilosclerida). Invertebr Biol 120(4):295–307
Vacelet J (1979) Quelques stades de la reproduction sexuel d'une éponge Sphinctozoaire actuelle. In: Lévi C, Boury-Esnault N (eds) Biologie des spongiaires. CNRS, Paris
Vogel S (1994) Life in moving fluids. The physical biology of flow. Princeton University Press, Princeton, N.J.
Walters LJ, Wethey DS (1996) Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: the importance of refuge dimensions and adult morphology. Mar Ecol Prog Ser 137:161–171
Weis VM, Keene DR, Buss LW (1985) Biology of hydractiniid hydroids. 4. Ultrastructure of the planula of Hydractinia echinata. Biol Bull 168:403–418
Wolken JJ (1995) Light detectors, photoreceptors, and imaging systems in nature. Oxford University Press, New York
Woollacott R (1990) Structure and swimming behavior of the larva of Halichondria melanodocia (Porifera: Demospongiae). J Morphol 205:135–145
Woollacott RM (1993) Structure and swimming behavior of the larva of Haliclona tubifera (Porifera: Demospongiae). J Morphol 218:301–321
Woollacott RM, Pinto RL (1995) Flagellar basal apparatus and its utility in phylogenetic analyses of the Porifera. J Morphol 226:247–265
Young CM, Chia F-S (1984) Microhabitat-associated variability in survival and growth of subtidal littoral ascidians during the first 21 d after settlement. Mar Biol 81:61–68
Acknowledgements
This study was supported by the Fulbright Association (FU-93-02207057) and two research grants from the Spanish government to M.M. (MEC-PB-98-0485; BMC-2002-01228). This is HBOI contribution number 1515 and SMSFP contribution number 563.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. A. Poulet, Roscoff
Rights and permissions
About this article
Cite this article
Maldonado, M., Durfort, M., McCarthy, D.A. et al. The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Marine Biology 143, 427–441 (2003). https://doi.org/10.1007/s00227-003-1100-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1100-1