Abstract
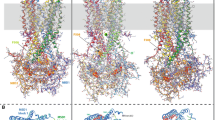
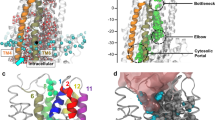
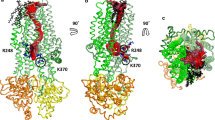
Cryo-electron microscopy (cryo-EM) has recently provided invaluable experimental data about the full-length cystic fibrosis transmembrane conductance regulator (CFTR) 3D structure. However, this experimental information deals with inactive states of the channel, either in an apo, quiescent conformation, in which nucleotide-binding domains (NBDs) are widely separated or in an ATP-bound, yet closed conformation. Here, we show that 3D structure models of the open and closed forms of the channel, now further supported by metadynamics simulations and by comparison with the cryo-EM data, could be used to gain some insights into critical features of the conformational transition toward active CFTR forms. These critical elements lie within membrane-spanning domains but also within NBD1 and the N-terminal extension, in which conformational plasticity is predicted to occur to help the interaction with filamin, one of the CFTR cellular partners.










Similar content being viewed by others
References
Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827–834
Gadsby DC (2009) Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol 10:344–352
Aleksandrov LA, Jensen TJ, Cui L, Kousouros JN, He L, Aleksandrov AA, Riordan JR (2015) Thermal stability of purified and reconstituted CFTR in a locked open channel conformation. Protein Expr Purif 116:159–166
Bozoky Z, Krzeminski M, Chong P, Forman-Kay JD (2013) Structural changes of CFTR R region upon phosphorylation: a plastic platform for intramolecular and intermolecular interactions. FEBS J 280:4407–4416
Callebaut I, Hoffmann B, Mornon J-P (2017) The implications of CFTR structural studies for cystic fibrosis drug development. Curr Opin Pharmacol 34:112–118
Zhang Z, Chen J (2016) Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell 167:1586–1597
Liu F, Zhang Z, Csanády L, Gadsby DC, Chen J (2017) Molecular structure of the human CFTR ion channel. Cell 169:85–95
Zhang Z, Liu F, Chen J (2017) Conformational changes of CFTR upon phosphorylation and ATP binding. Cell 170:483–491
Tordai H, Leveles I, Hegedus T (2017) Molecular dynamics of the cryo-EM CFTR structure. Biochem Biophys Res Commun 491:986–993
Corradi V, Gu R-X, Vergani P, Tieleman D (2018) Structure of transmembrane helix 8 and possible membrane defects in CFTR. Biophys J 114:1751–1754
Callebaut I, Hoffmann B, Lehn P, Mornon J-P (2017) Molecular modelling and molecular dynamics of CFTR. Cell Mol Life Sci 74:3–22
Dalton J, Kalid O, Schushan M, Ben-Tal N, Villà-Freixa J (2012) New model of cystic fibrosis transmembrane conductance regulator proposes active channel-like conformation. J Chem Inf Model 52:1842–1853
Norimatsu Y, Ivetac A, Alexander C, O’Donnell N, Frye L, Sansom MS, Dawson DC (2012) Locating a plausible binding site for an open-channel blocker, GlyH-101, in the pore of the cystic fibrosis transmembrane conductance regulator. Mol Pharmacol 82:1042–1055
Mornon J-P, Hoffmann B, Jonic S, Lehn P, Callebaut I (2015) Full-open and closed CFTR channels, with lateral tunnels from the cytoplasm and an alternative position of the F508 region, as revealed by molecular dynamics. Cell Mol Life Sci 72:1377–1403
Corradi V, Vergani P, Tieleman DP (2015) Cystic fibrosis transmembrane conductance regulator (CFTR): closed and open state channel models. J Biol Chem 290:22891–22906
Das J, Aleksandrov AA, Cui L, He L, Riordan JR, Dokholyan NV (2017) Transmembrane helical interactions in the CFTR channel pore. PLoS Comput Biol 13:e1005594
Linsdell P (2014) Functional architecture of the CFTR chloride channel. Mol Membr Biol 31:1–16
Cui G, Freeman CS, Knotts T, Prince CZ, Kuang C, McCarty NA (2013) Two salts bridges differentially contribute to the maintenance of cystic fibrosis transmembrane conductance regulator (CFTR) channel function. J Biol Chem 288:20758–20767
Cui G, Zhang ZR, O’Brien AR, Song B, McCarty NA (2008) Mutation at arginine 352 alters the pore architecture of CFTR. J Membr Biol 222:91–106
Cotten JF, Welsh MJ (1999) Cystic fibrosis-associated mutations at arginine 347 alter the pore architecture of CFTR. Evidence for disruption of a salt bridge. J Biol Chem 274:5429–5435
El Hiani Y, Linsdell P (2015) Functional architecture of the cytoplasmic entrance to the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 290:15855–15865
El Hiani Y, Negoda A, Linsdell P (2016) Cytoplasmic pathway followed by chloride ions to enter the CFTR channel pore. Cell Mol Life Sci 73:1917–1925
Gao X, Hwang TC (2015) Localizing a gate in CFTR. Proc Natl Acad Sci USA 112:2461–2466
Negoda A, El Hiani Y, Cowley EA, Linsdell P (2017) Contribution of a leucine residue in the first transmembrane segment to the selectivity filter region in the CFTR chloride channel. Biochem Biophys Acta 1859:1049–1058
Marti-Renom MA, Stuart A, Fiser A, Sánchez R, Melo F, Sali A (2000) Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct 29:291–325
Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S et al (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30:1545–1614
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29:1859–1865
Lee J, Cheng X, Swails JM, Yeom MS, Eastman PK, Lemkul JA, Wei S, Buckner J, Jeong JC, Yl Qi (2016) CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J Chem Theory Comput 12:405–413
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Lamoureux G, Roux B (2006) Absolute hydration free energy scale for alkali and halide ions established from simulations with a polarizable force field. J Phys Chem B 110:3308–3322
Beglov D, Roux B (1994) Finite representation of an infinite bulk system: solvent boundary potential for computer simulations. J Chem Phys 100:9050–9063
Hart K, Foloppe N, Baker CM, Denning EJ, Nilsson L, Mackerell AD (2012) Optimization of the CHARMM additive force field for DNA: improved treatment of the BI/BII conformational equilibrium. J Chem Theory Comput 8:348–362
Mackerell AD (2004) Empirical force fields for biological macromolecules: overview and issues. J Comput Chem 25:1584–1604
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K (2005) scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
Fletcher R (ed) (2000) Practical methods of optimization: fletcher/practical methods of optimization. Wiley, Chichester
Feller SE, Zhang Y, Pastor RW, Brooks BR (1995) Constant pressure molecular dynamics simulation: the Langevin piston method. J Chem Phys 103:4613–4621
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Laio A, Parrinello M (2002) Escaping free-energy minima. Proc Natl Acad Sci 99(99):12562–12566
Tribello GA, Bonomi M, Branduardi D, Camilloni C, Bussi G (2014) PLUMED 2: new feathers for an old bird. Comput Phys Commun 185:604–613
Pietrucci F (2017) Strategies for the exploration of free energy landscapes: unity in diversity and challenges ahead. Rev Phys 2:32–45
Branduardi D, Gervasio FL, Parrinello M (2007) From A to B in free energy space. J Chem Phys 126:054103
Crespo Y, Marinelli F, Pietrucci F, Laio A (2010) Metadynamics convergence law in a multidimensional system. Phys Rev E Stat Nonlinear Soft Matter Phys 81:055701
Johansson MU, Zoete V, Michielin O, Guex N (2012) Defining and searching for structural motifs using DeepView/Swiss-PdbViewer. BMC Bioinform 13:173
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Harrisson CB, Schulten K (2012) Quantum and classical dynamics simulations of ATP hydrolysis in solution. J Chem Theory Comput 8:2328–2335
de Meis L (1989) Role of water in the energy of hydrolysis of phosphate compounds—energy transduction in biological membranes. Biochem Biophys Acta 973:333–349
George P, Witonsky RJ, Trachtman M, Wu C, Dorwart W, Richman L, Richman W, Shurayh F, Lentz B (1970) “Squiggle-H2O”. An enquiry into the importance of solvation effects in phosphate ester and anhydride reactions. Biochem Biophys Acta 223:1–15
Vergani P, Nair AC, Gadsby D (2003) On the machanism of MgATP-dependent gating of CFTR Cl− channels. J Gen Physiol 121:17–36
Fay JF, Aleksandrov LA, Jensen TJ, Cui LL, Kousouros JN, He L, Aleksandrov AA, Gingerich DS, Riordan J, Chen JZ (2018) Cryo-EM visualization of an active high open probability CFTR ion channel. bioRxiv 274316
Alam A, Küng R, Kowal J, McLeod R, Tremp N, Broude E, Roninson I, Stahlberg H, Locher K (2018) Structure of a zosuquidar and UIC2-bound human-mouse chimeric ABCB1. Proc Natl Acad Sci USA 115:E1973–E1982
Kim Y, Chen J (2018) Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science 359:915–919
Johnson ZL, Chen J (2017) Structural basis of substrate recognition by the multidrug resistance protein MRP1. Cell 68:1075–1085
Hohl M, Hürlimann LM, Böhm S, Schöppe J, Grütter MG, Bordignon E, Seeger MA (2014) Structural basis for allosteric cross-talk between the asymmetric nucleotide binding sites of a heterodimeric ABC exporter. Proc Natl Acad Sci USA 111:11025–11030
Cooley RB, Arp DJ, Karplus PA (2010) Evolutionary origin of secondary structures: π-helices as cryptic but widespread insertional variations of α-helices that enhance protein functionality. J Mol Biol 404:232–246
Riek RP, Graham RM (2011) The elusive π-helix. J Struct Biol 173:153–160
Li M, Cowley E, El Hiani Y, Linsdell P (2018) Functional organization of cytoplasmic portals controlling access to the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel pore. J Biol Chem 293:5649–5658
Barreto-Ojeda E, Corradi V, Gu RX, Tieleman DP (2018) Coarse-grained molecular dynamics simulations reveal lipid access pathways in P-glycoprotein. J Gen Physiol. https://doi.org/10.1085/jgp.201711907
Dawson RJ, Locher KP (2006) Structure of a bacterial multidrug ABC transporter. Nature 443:180–185
Ward A, Reyes CL, Yu J, Roth CB, Chang G (2007) Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc Natl Acad Sci USA 104:19005–19010
Katzmann D, Epping E, Moye-Rowley W (1999) Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol Cell Biol 19:2998–3009
Göddeke H, Timachi M, Hutter C, Galazzo L, Seeger M, Karttunen M, Bordignon E, Schäfer L (2018) Atomistic mechanism of large-scale conformational transition in a heterodimeric ABC exporter. J Am Chem Soc 140:4543–4551
Locher KP (2016) Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol 23:487–493
Karpowich N, Martsinkevich O, Millen L, Yuan YR, Dai PL, MacVey K, Thomas PJ, Hunt JF (2001) Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure 9:571–586
Naoe Y, Nakamura N, Doi A, Sawabe M, Nakamura H, Shiro Y, Sugimoto H (2016) Crystal structure of bacterial haem importer complex in the inward-facing conformation. Nat Commun 7:13411
Woo J, Zeltina A, Goetz B, Locher K (2012) X-ray structure of the Yersinia pestis heme transporter HmuUV. Nat Struct Mol Biol 19:1310–1315
Wang C, Karpowich N, Hunt J, Rance M, Palmer A (2004) Dynamics of ATP-binding cassette contribute to allosteric control, nucleotide binding and energy transduction in ABC transporters. J Mol Biol 342:525–537
Li N, Wu JX, Ding D, Cheng J, Gao N, Chen L (2017) Structure of a pancreatic ATP-sensitive potassium channel. Cell 168:101–110
Martin GM, Yoshioka C, Rex EA, Fay JF, Xie Q, Whorton MR, Chen JZ, Shyng SL (2017) Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Elife 6:e24149
Thelin WR, Chen Y, Gentzsch M, Kreda SM, Sallee JL, Scarlett CO, Borchers CH, Jacobson K, Stutts MJ, Milgram SL (2007) Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J Clin Investig 117:364–374
Feng Y, Walsh CA (2004) The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 6:1034–1038
Nakamura F, Stossel TP, Hartwig JH (2011) The filamins. Cell Adhes Migr 5:160–169
Playford MP, Nurminen E, Pentikäinen OT, Milgram SL, Hartwig JH, Stossel TP, Nakamura F (2010) Cystic fibrosis transmembrane conductance regulator interacts with multiple immunoglobulin domains of filamin A. J Biol Chem 285:17156–17165
Smith L, Page RC, Xu Z, Kohli E, Litman P, Nix J, Ithychanda SS, Liu J, Qin J, Misra S et al (2010) Biochemical basis of the interaction between cystic fibrosis transmembrane conductance regulator and immunoglobulin-like repeats of filamin. J Biol Chem 285:17166–17176
Light S, Sagit R, Ithychanda SS, Qin J, Elofsson A (2015) The evolution of filamin-a protein domain repeat perspective. J Struct Biol 179:289–298
Sampson LJ, Leyland ML, Dart C (2003) Direct interaction between the actin-binding protein filamin-A and the inwardly rectifying potassium channel, Kir2.1. J Biol Chem 278:41988–41997
Sethi R, Seppälä J, Tossavainen H, Ylilauri M, Ruskamo S, Pentikäinen OT, Pentikäinen U, Permi P, Ylänne J (2014) A novel structural unit in the N-terminal region of filamins. J Biol Chem 289:8588–8598
Gaboriaud C, Bissery V, Benchetrit T, Mornon JP (1987) Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett 224:149–155
Callebaut I, Labesse G, Durand P, Poupon A, Canard L, Chomilier J, Henrissat B, Mornon J-P (1997) Deciphering protein sequence information through hydrophobic cluster analysis (HCA): current status and perspectives. Cell Mol Life Sci 53:621–645
Bitard-Feildel T, Callebaut I (2017) Exploring the dark foldable proteome by considering hydrophobic amino acids topology. Sci Rep 7:41425
Eudes R, Le Tuan K, Delettré J, Mornon J-P, Callebaut I (2007) A generalized analysis of hydrophobic and loop clusters within globular protein sequences. BMC Struct Biol 7:2
Rebehmed J, Quintus F, Mornon J-P, Callebaut I (2016) The respective roles of polar/nonpolar binary patterns and amino acid composition in protein regular secondary structures explored exhaustively using hydrophobic cluster analysis. Proteins 84:624–638
Wang W, He Z, O’Shaughnessy TJ, Rux J, Reenstra WW (2002) Domain-domain associations in cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 282:C1170–C1180
Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE et al (2016) From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 27:424–433
Chang SY, Di A, Naren AP, Palfrey HC, Kirk KL, Nelson DJ (2002) Mechanisms of CFTR regulation by syntaxin 1A and PKA. J Cell Sci 115:783–791
Cormet-Boyaka E, Di A, Chang SY, Naren AP, Tousson A, Nelson DJ, Kirk KL (2002) CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex. Proc Natl Acad Sci USA 99:12477–12482
Li C, Roy K, Dandridge K, Naren AP (2004) Molecular assembly of cystic fibrosis transmembrane conductance regulator in plasma membrane. J Biol Chem 279:24673–24684
Ameen N, Silvis M, Bradbury NA (2007) Endocytic trafficking of CFTR in health and disease. J Cyst Fibros 6:1–14
Peters KW, Qi J, Johnson JP, Watkins SC, Frizzell RA (2001) Role of snare proteins in CFTR and ENaC trafficking. Pflugers Arch 443:S65–S69
Ford RC (2016) ABC7/CFTR. In: Goerge A (ed) ABC transporters—40 years on. Springer International Publishing, New York, pp 319–340
Naren AP, Cormet-Boyaka E, Fu J, Villain M, Blalock JE, Quick MW, Kirk KL (1999) CFTR chloride channel regulation by an interdomain interaction. Science 286:544–548
Protasevich I, Yang Z, Wang C, Atwell S, Zhao X, Emtage S, Wetmore D, Hunt J, Brouillette CG (2010) Thermal unfolding studies show the disease causing F508del mutation in CFTR thermodynamically destabilizes nucleotide-binding domain 1. Protein Sci 19:1917–1931
Wang C, Protasevich I, Yang Z, Seehausen D, Skalak T, Zhao X, Atwell S, Spencer Emtage J, Wetmore DR, Brouillette CG et al (2010) Integrated biophysical studies implicate partial unfolding of NBD1 of CFTR in the molecular pathogenesis of F508del cystic fibrosis. Protein Sci 19:1932–1947
He L, Aleksandrov AA, An J, Cui L, Yang Z, Brouillette CG, Riordan JR (2015) Restauration of NBD1 thermal stability is necessary and sufficient to correct DF508 CFTR folding and assembly. J Mol Biol 427:106–120
Bakos E, Evers R, Szakács G, Tusnády GE, Welker E, Szabó K, de Haas M, van Deemter L, Borst P, Váradi A et al (1998) Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem 273:32167–32175
Furini S, Domene C (2016) Computational studies of transport in ion channels using metadynamics. Biochim Biophys Acta 1858:1733–1740
Sheppard DN, Rich DP, Ostedgaard LS, Gregory RJ, Smith AE, Welsh MJ (1993) Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature 362:160–164
Yu YC, Sohma Y, Hwang TC (2016) On the mechanism of gating defects caused by the R117H mutation in cystic fibrosis transmembrane conductance regulator. J Physiol 594:3227–3244
Sorum B, Töröcsik B, Csanády L (2017) Asymmetry of movements in CFTR’s two ATP sites during pore opening serves their distinct functions. Elife 6:e29013
Sorum B, Czégé D, Csanády L (2015) Timing of CFTR pore opening and structure of its transition state. Cell 163:724–733
Acknowledgements
This work was funded by the French Association Vaincre La Mucoviscidose (Paris). It was granted access to the HPC resources of IDRIS/CINES under the allocations 2014-077206, 2015-077206, 2016-077206, and 0020707206 made by GENCI.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoffmann, B., Elbahnsi, A., Lehn, P. et al. Combining theoretical and experimental data to decipher CFTR 3D structures and functions. Cell. Mol. Life Sci. 75, 3829–3855 (2018). https://doi.org/10.1007/s00018-018-2835-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2835-7